Research Interests
Pharmacology and Aging: Mechanism and Intervention
Brain Aging: neurodegenerative diseases including AD and drug discovery
Bone Aging: biomineralization and osteoporosis
Body Aging: mechanisms by natural small-molecular products
Functional research of the spermatogenesis-related gene SPATA4 on aging
Molecular & Cellular Pharmacology of Natural Products on aging and related diseases
R&D of traditional Chinese herbal medicine for clinical drugs and functional food
Scientific Contributions
Recent years, our research interests are focusing on neuropharmacology and metabolic pharmacology, especially the pharmacology related with brain aging, bone aging and degenerative diseases such as AD and osteoporosis. The pathology of AD is characterized by amyloid deposits in limited regions of the brain, such as the entorhinal cortex, hippocampus and basal forebrain. However, it is interesting that the cerebellum is spared from significant amyloid-b (Ab) accumulation. This discovery aroused our great interest. We highlighted our work on the role of metabolites of neurons from the different region of the brain in the pathogenesis of AD, and discovered that the metabolites of cerebellar neurons could combat pathogenesis of AD, while the metabolites of hippocampal neurons could facilitate it. These exciting findings bring forward a novel insight with the possible new targets in the clinical therapy for AD patients. As the mechanism, our results reveal for the first time that PPARg could directly interact with IDE gene promoter and regulate IDE expression through binding to a functional peroxisome proliferator-response element (PPRE) in IDE promoter thus promoting IDE gene transcription. Because the greatest known risk factor for AD is increasing age, our findings of differential alteration of PPARg activity in cerebellum and hippocampus could at least partially explain the regional specificity of AD pathology.
Another research interest is Bone aging especially Bone Biomineralization and Osteoporosis. We Identified and characterized a serial biomineralization related genes PFMG1~10 highly expressed in the mantle of
pinctada fucata. So far our results showed that most of PFMGs revealed upregulation of the marker genes related to cell growth, differentiation, and mineralization, and BMP-2, osterix, and osteopontin were upregulated. Some of PFMGs could promote differentiation in mouse osteoblasts while PFMG5 inhibits osteoblast differentiation. For example, PFMG1 promotes osteoblast proliferation and affects cell cycle distribution. PFMG1 accelerates osteoblast differentiation and matrix mineralization through activating Erk-Runx2 signaling pathway. PFMG1 also accelerates calcium crystals aggregation in culture medium and suppresses osteoclast formation. Moreover, PFMG1 prevents bone loss caused by ovariectomy without affecting cortical bone. Our findings showed the therapeutic potential of protein PFMG1 from nacre as a novel drug for osteoporosis.
Another study showed that exogenous cAMP can improve aging-related phenotypes via increasing the protein level of Sirtuin, which prevented metabolic disorders to mimic the effect of calorie restriction, suggesting that cAMP slow the aging process and is a good candidate to mimic calorie restriction. Our research provided a promising therapeutic strategy for the intervention of metabolic disorder induced aging-related diseases.
Selected Achievements
Discovered that the metabolites of cerebellar neurons could combat pathogenesis of AD, while the metabolites of hippocampal neurons could facilitate it. These exciting findings bring forward a novel insight with the possible new targets in the clinical therapy for AD patients.
The regulation of IDE by PPAR
g and their roles in AD and DM2 pathology are illustrated. Upon activation by its agonists (e.g., TZDs), cytosolic PPARg is translocated to the nucleus, where it binds to PPRE in IDE gene promoter region and induces IDE gene transcription. Up-regulated IDE then promotes the clearance of Ab and insulin, which could protect against AD and DM2, respectively.
Discovered that the metabolites of cerebellar neurons could improve the learning and memory functions in APP/PS1 transgenic mice.
In vivo metabolites of cerebellar neurons reversed Alzheimer’s disease-like phenotypes of APP/PS1 transgenic mice.
Discovered a serial biomineralization related matrix protein PFMGs, from the mantle of
pinctada fucata, could promote osteoblast proliferation and affects cell cycle distribution, accelerate osteoblast differentiation and matrix mineralization. Most of PFMGs also accelerate calcium crystals aggregation in culture medium and suppresses osteoclast formation, prevent bone loss caused by ovariectomy, suggesting the therapeutic potential of matrix proteins rom nacre as a novel drug for osteoporosis.
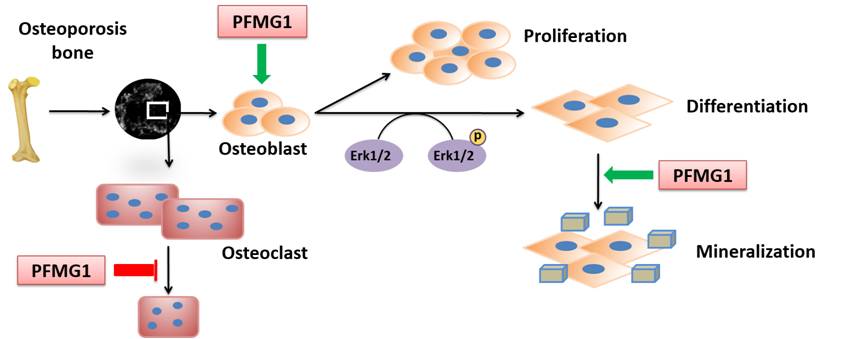
Schematic diagram of PFMG1 in regulating bone remodeling after the bone loss caused by ovariectomy. PFMG1 accelerates osteoblast proliferation and promotes osteoblast differentiation through activation of the Erk signaling pathway. PFMG1 may also inhibit osteoclast development
Discovered exogenous cAMP can improve aging-related phenotypes, suggesting that cAMP slow the aging process and is a good candidate to mimic calorie restriction, which provided a promising therapeutic strategy for the intervention of metabolic disorder induced aging-related diseases.
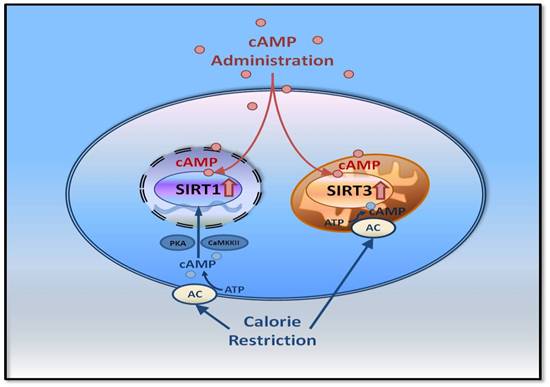
Graphical Abstract for the Underlying Mechanism of the cAMP Regulation Effect on Sirtuin.
Professional Membership/Social Activities
Senior Member, Chinese Pharmaceutical Association
Member, Chinese Pharmacological Society
Member of the Editorial Board,
Pharmazie (Germany)
Member of the Editorial Board,
Journal of Pharmacological Sciences (Japan)
Referendary Expert of the Functional Food, CFDA
Editor,
Food Science and Human Wellness (China)
Special supervisor, People's Government of Beijing Municipality






