Research Interests
We are interested in studying the immune regulatory mechanisms during tumor development and metastasis, as well as their roles in cancer immunotherapy. Our ultimate goal is to identify new targets and to develop new therapeutics for cancer immunotherapy. Specifically, our studies include: 1> to identify new drivers of tumor development or evasion; 2> to define the roles of immunoregulatory pathways in tumor microenvironment and immunotherapy; 3> to develop new next-generation antibody for cancer immunotherapy.
Major Scientific Contribution
1. Our studies show that sufficient lymphocyte infiltration is essential for the response to checkpoint blockade therapy. We develop multiple unique next-generation antibody therapeutics, which are able to enhance the activation of T cells and antigen presentation by dendritic cells. Such therapeutics are able to increase the efficacy of immune checkpoint blockade therapy (Cancer Cell, 2016; Nat Commun, 2018). These studies provide potential new strategies to boost the current immunotherapy, and to overcome tumor resistant to the current therapy.
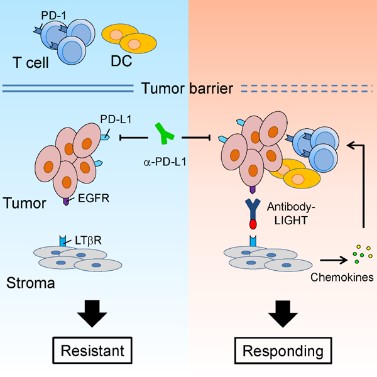
2. It is commonly believed that PD-L1 on tumor cells interacts with PD-1 on T cells and suppresses T cell responses, leading to tumor immune evasion. However, clinical benefits from immune checkpoint blockade therapy are observed in some patients that are negative for PD-L1 in tumors. Our studies show that PD-L1 is highly expressed on myeloid cells, especially dendritic cells. PD-L1 on dendritic cells negatively regulates antigen presentation, thus inhibits T cell activation (Nat Commun, 2020; J Clin Invest, 2018). PD-L1 expression in tumor associated myeloid cells might be a better biomarker to predict the responsiveness to immune checkpoint blockade therapy.
Honors and Awards
·2019, Bayer Investigator Award
·2014, Irvington Postdoctoral Fellowship, Cancer Research Institute (New York)
·2012, Sanofi Pasteur-BIOLS Biomedicine Award for Outstanding Graduate Students
Selected publications
(#co-first author, *corresponding author)
1. Peng Q, Qiu X, Zhang Z, Zhang S, Zhang Y, Liang Y, Guo J, Peng H, Chen M, Fu Y-X, Tang H*. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat Commun. 2020, 11: 4835.
2. Zuo H, Yang D, Yang Q, Tang H, Fu Y-X, Wan Y. Differential regulation of breast cancer bone metastasis by PARP1 and PARP2. Nat Commun. 2020, 11: 1578.
3. Tang H#, Liang Y#, Anders R, Taube J, Qiu X, Mulgaonkar A, Liu X, Harrington S, Guo J, Xin Y, Xiong Y, Nham K, Silvers W, Hao G, Sun X, Chen M, Hannan R, Qiao J, Peng H, Dong H, Fu Y-X. PD-L1 on host cells is essential for tumor regression mediated by PD-L1 blockade. J Clin Invest. 2018, 128: 580-588.
4. Tang H*, Qiu X, Timmerman C, Fu Y-X. Targeting tertiary lymphoid structures for tumor immunotherapy. Methods Mol Biol. 2018, 1845: 275-286.
5. Tang H, Fu Y-X. Immune Evasion in Tumor’s Own Sweet Way. Cell Metab. 2018, 27: 945-946.
6. Liang Y#, Tang H#*, Guo J#, Qiu X, Ren Z, Bian Y, Dong H, Peng H*, Fu Y-X*. Targeting type I interferon into tumor by anti-PD-L1 creates feedforward antitumor responses to overcome innate and adaptive resistance. Nat Commun. 2018, 9: 4586.
7. Lu K, He C, Guo N, Chan C, Ni K, Lan G, Tang H, Pelizzari C, Fu Y-X, Spiotto M, Weichselbaum R, Lin W. Low-dose X-ray radiotherapy-radiodynamic therapy via nanoscale metal-organic frameworks enhances checkpoint blockade immunotherapy. Nat Biomed Eng. 2018, 2: 600-610.
8. Deng M, Gui X, Kim J, Xie L, Chen W, Li Z, He L, Chen Y, Chen H, Luo W, Lu Z, Xie J, Churchill H, Xu Y, Zhou Z, Wu G, Yu C, John S, Hirayasu K, Nguyen N, Liu X, Huang F, Li L, Deng H, Tang H, Sadek AH, Zhang L, Huang T, Zou Y, Chen B, Zhu H, Arase H, Xia N, Jiang Y, Collins R, You MJ, Homsi J, Unni N, Lewis C, Chen GQ, Fu YX, Liao XC, An Z, Zheng J, Zhang N, Zhang CC. LILRB4 signalling in leukaemia cells mediates T cell suppression and tumour infiltration. Nature. 2018, 562: 605-609.
9. Zhang Y, Kim T-J, Wroblewska J, Tesic V, Upadhyay V, Weichselbaum R, Tumornoy A, Tang H, Guo X, Tang H, Fu Y-X. Type 3 innate lymphoid cell-derived lymphotoxin prevents microbiota-dependent inflammation. Cell Mol Immunol. 2017, 14: 1-13.
10. Tang H*, Zhu M, Qiao J, Fu Y-X*. Lymphotoxin signaling in tertiary lymphoid structures and immunotherapy. Cell Mol Immunol. 2017, 14: 809-818.
11. Qiao J, Tang H, Fu Y-X. DNA sensing and immune responses in cancer therapy. Curr Opin Immunol. 2017, 45: 16-20.
12. Wroblewska J, Zhang Y, Tang H, Guo X, Nagler C, Fu Y-X. Cutting Edge: Lymphotoxin Signaling Is Essential for Clearance of Salmonella from the Gut Lumen and Generation of Anti-Salmonella Protective Immunity. J Immunol. 2017, 198: 55-60.
13. Tang H, Wang Y, Chlewicki L, Zhang Y, Guo J, Liang W, Wang J, Wang X, Fu Y-X. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell. 2016, 29 (3): 285-296.
14. Tang H, Qiao J, Fu Y-X. Immunotherapy and tumor microenvironment. Cancer Lett. 2016, 370: 85-90.
15. Okwor I, Xu G, Tang H, Liang Y, Fu Y-X, Uzonna J. Deficiency of CD40 reveals an important role for LIGHT in anti-Leishmania immunity. J Immunol. 2015, 195: 194-202.
16. Li C, Yang Z, Du Y, Tang H, Chen J, Hu D, Fan Z. BCMab1, A monoclonal antibody against aberrantly glycosylated integrin a3b1, has potent antitumor activity of bladder cancer in vivo. Clin Cancer Res, 2014, 20: 4001-4013.
17. Tang H, Li C, Wang L, Zhang H, Fan Z. Granzyme H of cytotoxic lymphocytes is required for clearance of the hepatitis B virus through cleavage of the hepatitis B virus X protein. J Immunol.2012, 188: 824-831.
18. Yang Z, Tang H, Huang H, Deng H. RTA promoter demethylation and histone acetylation regulation of murine gamma-herpesvirus 68 reactivation. PLoS One. 2009, 4 (2): e4556.






