Research interests
(1) Drug design and delivery against pancreatic cancer
Pancreatic cancer is known as the "king of cancers" and there is still a serious lack of effective therapeutic agents. The unique physiology, tumor biology, and tumor immune microenvironment of pancreatic cancer contribute to this dilemma. We are committed to understanding and exploiting the mechanisms of albumin transport and metabolism in pancreatic cancer, in order to design new drugs and drug delivery strategies for pancreatic cancer treatment. At the same time, we also explore the possibility of using some natural products with unique pharmacological mechanisms for pancreatic cancer therapy.
(2) Drug design and delivery for retinal diseases
As the population continues to age, blinding retinal diseases such as age-related macular degeneration, diabetic macular edema, and glaucoma continue to climb. The most effective mode of drug delivery for these fundus diseases is intravitreal injection. However, the molecular design and delivery of drugs greatly affects the pharmacological activity, potency, injectability, long-term stability, and other key characteristics of such drugs, thereby greatly affecting efficacy, compliance, and ultimately control of disease progression. We strive to understand the relationship between structure and function of drug targets, explore the influence of protein drug structure on their solution and pharmacokinetic properties, based on which we are engineering novel protein drugs for the efficient treatment of multiple fundus diseases.
(3) Physical pharmaceutics and biopharmaceutics of various types of drugs
As new drug types continue to evolve and iterate, we continue to study drug-drug, drug-excipient, and formulation-bio-tissue interactions for various classes of drugs, such as poorly soluble and difficult-to-absorb (BCS 2/3/4 “beyond-rule-of-five” drugs, including various small molecules, peptides, etc.), antibodies, and fusion proteins. Furthermore, we investigate the effects of these interactions on the physical stability, oral bioavailability, absorption pathways, and other physical and biopharmaceutical properties of the various classes of drug formulations.
Scientific Contributions
1. Influence of "drug-material-water" intermolecular interactions on the structure and behavior of formulations:
As a thermodynamically unstable system, the physical stability of amorphous drug-polymer solid dispersion (ASD) is one of the main risks of this formulation. We propose a working roadmap for designing ASDs to reduce the stability risk of ASDs. This idea is widely recognized by industry peers. In addition, we propose that the intermolecular interactions of drug-material-water are the central influencing factors in determining the pharmaceutical performance of ASDs, and we have established a methodology to characterize the drug-material-water interactions. We have also clearly demonstrated the effect of drug-material interactions on the dissolution and bioavailability of drug formulations through in vivo and ex vivo experiments. These works are of great significance for the design and optimization of difficult-to-dissolve drug formulations.
2. Drug delivery strategies for targeting cancer cells with KRAS mutations:
KRAS mutations are considered difficult for targeted drug design. We propose a drug delivery strategy for targeting KRAS mutant cancer cells. This strategy is proposed based on the biological characteristics of KRAS mutant cancer cells that differ from KRAS wild-type normal cells, i.e., enhanced macropinocytosis and low expression of FcRn. Within the field of drug delivery, it is a novel attempt to utilize disease biology mechanisms to target drug delivery to specific genotype cells.
3. Aggregation kinetics and solution stabilization mechanisms for highly concentrated proteins:
Subcutaneous and local injections of protein drugs often require very highly concentrated protein solution formulations. At high concentrations, protein molecular interactions become more complex, which can negatively affect both structural and colloidal stability of proteins and accelerate irreversible protein aggregation, leading to decreased efficacy, decreased apparent product quality, and decreased safety of the medication due to allergic reactions in patients. The design and risk evaluation of highly concentrated protein solutions is generally done in a trial-and-error manner. We try to investigate the key factors and kinetic processes affecting the phase separation and aggregation of protein solutions at the level of physicochemical and colloidal sciences to provide a theoretical basis for the design of highly concentrated protein formulations.
Selected Achievements
1. Impact of "drug-material-water" intermolecular interactions on the pharmacological behavior of formulations:
We proposed in 2015 a methodology to study the "drug-material-water", "χASD-water plot" (Flory-Huggins interaction parameters of ASD and water relative to the drug loading of the ASD , Figure 1), we found that this characterization tool can clearly reveal the interaction strengths, types, and changes in the interaction between drugs and macromolecules in ASDs in the presence of water, and found that these intermolecular interactions are closely related to the dissolution of ASDs. ASD showed optimal dissolution when drug-polymer interactions made the system more hydrophobic and this interaction was not disrupted by water. This work confirmed for the first time the association between intermolecular interactions and the dissolution behavior of formulations. Subsequently, we have validated the effect of intermolecular interactions between drugs and materials on the structure and behavior of formulations in several studies, and this line of molecular pharmaceutics research has supported the formulation development of several new drugs. (Mol. Pharmaceutic, 2016, 13, 599–608; Mol. Pharmaceutic, 2016, 13, 2787-2795;Pharm Res, 2016, 33(10), 2445-2458;Cryst. Growth Des., 2016, 16, 5367-5376;Mol. Pharmaceutics, 2018, 15 (7), 2754–2763;Mol. Pharmaceutics, 2019, 16(1), pp 318–326; ACS Infect. Dis. 2020, 6, 5, 802–810;etc.)
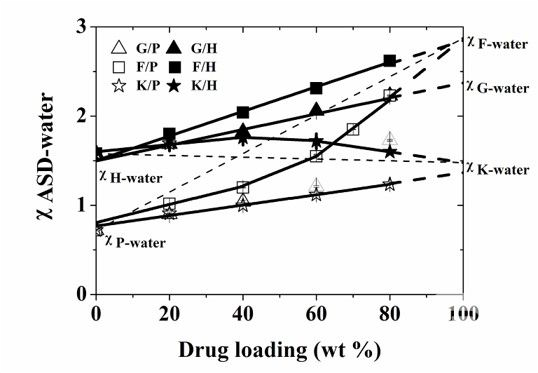
Fig. 1. Relationship between χASD-water and ASD loading for six ASD systems (G, F, and K for three model drugs, and P, H for two gauzons, PVP-VA and HPMC-AS, respectively). This illustration predicts the dissolution performance of different ASDs, thus providing clear theoretical guidance for the design of ASD formulations. (Molecular Pharmaceutics, 2015, 12(2):576-89.)
2. Drug delivery for KRAS-mutant cancers:
Although medicinal chemists have made considerable progress in designing inhibitors targeting KRAS, mutant KRAS (especially G12D, G12V, etc.) are still considered to be difficult targets for drug development. We propose a drug delivery strategy against KRAS mutant cancer cells. Based on the enhanced macrocytosis and low FcRn receptor expression in KRAS-mutant cells, we found that albumin-conjugated drugs, while maintaining the native structure of albumin, will significantly rely on macropinocytosis to enter KRAS-mutant cancer cells, and efficiently release their drug payloads through the lysosomal degradation pathway in the absence of FcRn-binding protection. In contrast to KRAS-mutant pancreatic cancer cells, KRAS wild-type normal epithelial cells take up the albumin-conjugated drug in a clathrin-mediated endocytosis manner, and then are rapidly sorted into the circulating vesicles and subsequently underwent exocytosis due to their tight binding to the FcRn. We found that benefiting from this difference in endocytosis transport phenotype under different KRAS genotypes, albumin-conjugated drugs can significantly increase (∼10-fold) the therapeutic window. This strategy of albumin-conjugation offers the possibility of obtaining KRAS cellular targeting by means of drug delivery (Fig. 2). We also explored other drug delivery systems with KRAS-targeting features, as well as drug delivery systems targeting the microenvironment of KRAS-mutant pancreatic cancer tumors. Based on the relevant findings, we are exploring the development of a series of novel drug delivery strategies targeting KRAS-mutant tumors, particularly pancreatic cancers. (Cancer Letters, 2022,539, 215718;Theranostics. 2022; 12(3): 1061-1073;J Control Release. 2020;323(March):311-320;Advanced Therapeutics. 2019, 1900032, ACS Nano, 2019 23;13(4):4049-4063; Small, 2018 Dec;14(51):e1802112,etc.)
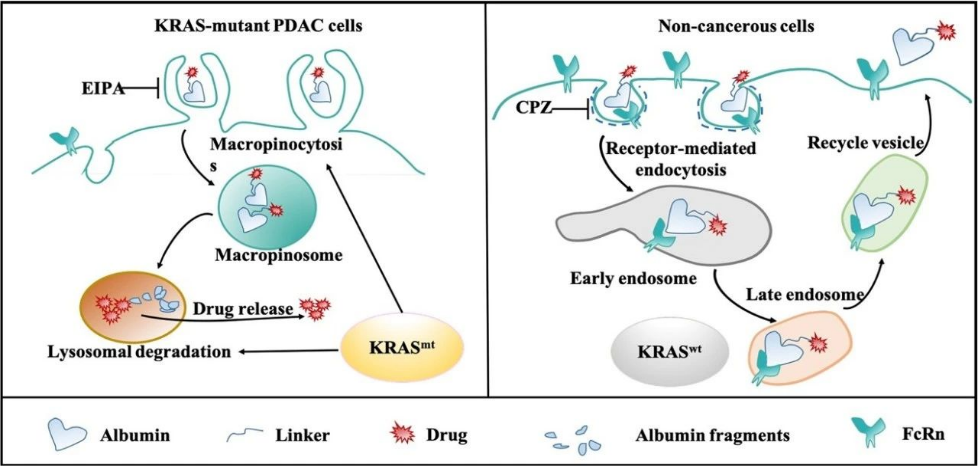
Fig. 2. Schematic representation of the endocytic transport mechanism of albumin drug conjugates.
3. Aggregation kinetics and solution stability mechanisms of highly concentrated proteins
Protein drugs represented by monoclonal antibody (mAb) are one of the most rapidly developing classes of biomolecules, with advantages such as strong targeting and few side effects. Due to the high dosage of mAb and the demand for subcutaneous and local (e.g., intravitreal) injection of small volume, its formulation is often a high-concentration colloidal solution, which makes the interactions of mAb molecules in the preparation become more complex, and can have a negative impact on the structure and colloidal stability of mAb and accelerate the irreversible aggregation of mAb, which can lead to a decrease in the efficacy and apparent quality, and increase the safety risks. We are committed to investigating the kinetic basis and stability mechanisms of monoclonal antibody aggregation. The monoclonal antibody molecules were found to nucleate and grow to form highly concentrated monoclonal antibody droplets with first-order kinetics during the phase separation process, and eventually settle to form a distinct phase separation state. In this study, quantifiable key kinetic parameters such as nucleation rate, growth rate, and onset of nucleation were defined from the perspective of monoclonal antibody aggregation kinetics, which were used to evaluate the rate of monoclonal antibody aggregation in solution (Figure 3).
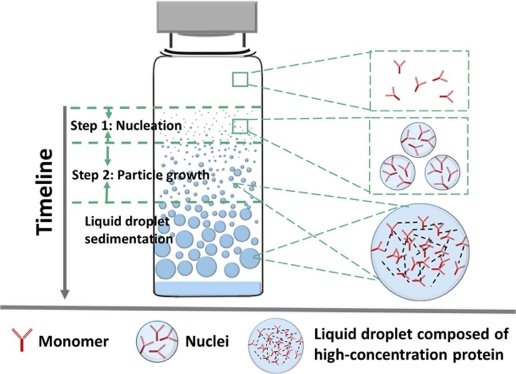
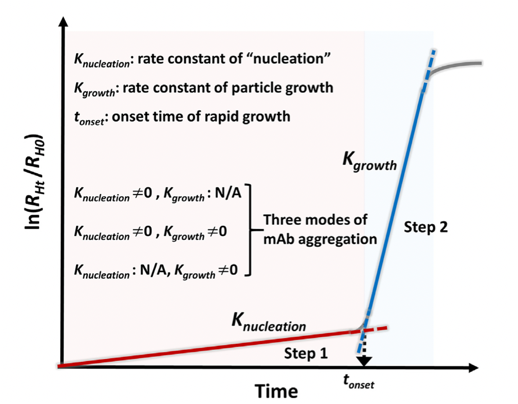
Figure 3. Kinetic process of phase separation of monoclonal antibody solution: nucleation and growth (International Journal of Pharmaceutics, 2020, 588, 119681.)
We have also investigated the mode of protein-protein interactions, and protein-excipient interactions, and found that minor excipient changes in protein solution formulations have the potential to significantly alter the mode and strength of protein interactions in solution formulations, thereby inducing a range of protein solution formulation stability risks such as liquid-liquid phase separation (Molecular Pharmaceutics, 2021, 18, 1, 267-274). These findings provide helpful theoretical basis for the development of highly concentrated protein formulations.
Honors and awards
2014-2015 Janssen-Tsinghua Investigator
2008 “Chemistry Leadership Award”, Bristol-Myers Squibb Company, New Jersey, USA
Selected Publications
1. Dou L, Liu H., Wang K, Liu J, Liu L, Ye J, Wang R, Deng H, Qian F.*; Albumin binding revitalizes NQO1 bioactivatable drugs as novel therapeutics for pancreatic cancer, Journal of Controlled Release, 2022 Aug 4;349:876-889. doi: 10.1016/j.jconrel.2022.07.033.
2. Kong W, Liu Z, Sun M, Liu H, Kong C, Ma J, Wang R, Qian F.*; Synergistic Autophagy Blockade and VDR Signaling Activation Enhance Stellate Cell Reprogramming in Pancreatic Ductal Adenocarcinoma, Cancer Letters. 2022 Jul 28;539:215718. doi: 10.1016/j.canlet.2022.215718. Epub 2022 May 5.
3. Fang Yuan, Mengnan Sun, Zhengsheng Liu, Huiqin Liu, Weijian Kong, Rui Wang, Qian F*, Macropinocytic dextran facilitates KRAS-targeted delivery while reducing drug-induced tumor immunity depletion in pancreatic cancer. Theranostics. 2022; 12(3): 1061-1073. doi: 10.7150/thno.65299
4. Liu H; Yu S; Qian F*, Opportunities and delusions regarding drug delivery targeting pancreatic cancer-associated fibroblasts, Advanced Drug Delivery Reviews, 2021 May;172:37-51. https://doi.org/10.1016/j.addr.2021.02.012
5. Zhou T,; Qian F.*; Adenosine Triphosphate-Induced Rapid Liquid–Liquid Phase Separation of a Model IgG1 mAb, Mol. Pharmaceutics. 2021 18 (1), 267-274, DOI: 10.1021/acs.molpharmaceut.0c00905
6. Mi W; Chen H; Zhu A, Zhang T, Qian F*, Melting point prediction of organic molecules by deciphering the chemical structure into a natural language, Chemical Communications, 2021, 57, 2633 – 2636
7. Yuan F; Sun M; Liu H; Qian, F.*, Albumin-conjugated drug is irresistible by single gene mutation of endocytic system: verification by genome-wide CRISPR-Cas9 loss-of function screens, Journal of Controlled Release, Vol 323, 10 July 2020, Pages 311-320
8. Hu C.; Liu Z.; Liu C.; Zhang Y.; Fan H.; Qian F.*; Improvement of Antialveolar Echinococcosis Efficacy of Albendazole by a Novel Nanocrystalline Formulation with Enhanced Oral Bioavailability, ACS Infect. Dis. 2020, 6, 5, 802–810
9. Kong C; Li Y; Liu Z; Ye J; Wang Z; Zhang L; Kong W.; Liu H.; Liu C.; Pang H.; Hu Z.; Gao J.; and Qian, F*, Targeting the Oncogene KRAS Mutant Pancreatic Cancer by Synergistic Blocking of Lysosomal Acidification and Rapid Drug Release, ACS Nano, DOI: 10.1021/acsnano.8b08246, March 26, 2019.
10. Liu H; Sun M; Liu Z; Kong C; Kong W; Ye J; Gong J; Huang D; Qian, F.*, KRAS-enhanced macropinocytosis and reduced FcRn-mediated recycling sensitize pancreatic cancer to albumin-conjugated drugs, Journal of Controlled Release, Vol 296, 28 February 2019, Pages 40-53.






