Research Overview:
The discovery and functional validation of novel drug targets remains a fundamental challenge in innovative drug development. Increasing evidence indicates that metabolic dysregulation not only underlies classical metabolic disorders but also plays a central role in the pathogenesis and progression of complex diseases, including cancer, immune disorders, and heart failure. In particular, the tumor microenvironment (TME) has emerged as a key regulatory niche, where intricate metabolic interactions among cancer cells, immune cells, and neurons form dynamic networks that shape disease trajectory and therapeutic response.
Of growing interest is the critical role of the nervous system in orchestrating tumor progression, metastasis, and therapy resistance. The metabolism-driven tumor–nerve–immune axis represents a frontier area in cancer biology, offering new insights into disease mechanisms and opportunities for therapeutic intervention.
The Hu Lab is dedicated to investigating the mechanisms of metabolic reprogramming in cancer and other major diseases, with an emphasis on identifying novel drug targets and developing precision therapeutic strategies. Our research integrates cutting-edge single-cell metabolomics, AI-assisted multi-omics data integration, and systems-level biological approaches, including bioinformatics, molecular biology, and cell biology, to dissect metabolic heterogeneity and intercellular communication within complex tissue microenvironments.
Key Research Areas:
1. Multicellular Metabolic Interactions and Single-Cell Metabolic Heterogeneity in the TME
We apply high-sensitivity micro-scale metabolomics and single-cell multi-omics to map intercellular metabolic interactions across diverse cell types—including cancer cells, immune cells, and neurons—within the TME. Particular emphasis is placed on elucidating the tumor–nerve–immune metabolic axis, focusing on the regulatory roles of neurotransmitters and neuropeptides in shaping microenvironmental metabolic states and identifying druggable nodes.
2. Metabolic Reprogramming and Adaptation in Drug-Tolerant Persister (DTP) Cells
We investigate how DTP states emerge and evolve under therapeutic pressure, focusing on metabolic adaptations and their interactions with neural components and immune cells in the TME. This aims to identify metabolic vulnerabilities and develop strategies to overcome drug resistance.
3. Development of Cross-Scale Metabolomics and AI-Enabled Multi-Omics Platforms
We develop high-sensitivity, broad-coverage metabolomics and metabolic flux profiling technologies tailored for low-input and single-cell analysis. These tools are integrated with AI-driven computational pipelines to enable multi-layered data integration across transcriptomics, proteomics, and metabolomics, supporting systems-level modeling, target discovery, and functional validation in disease.
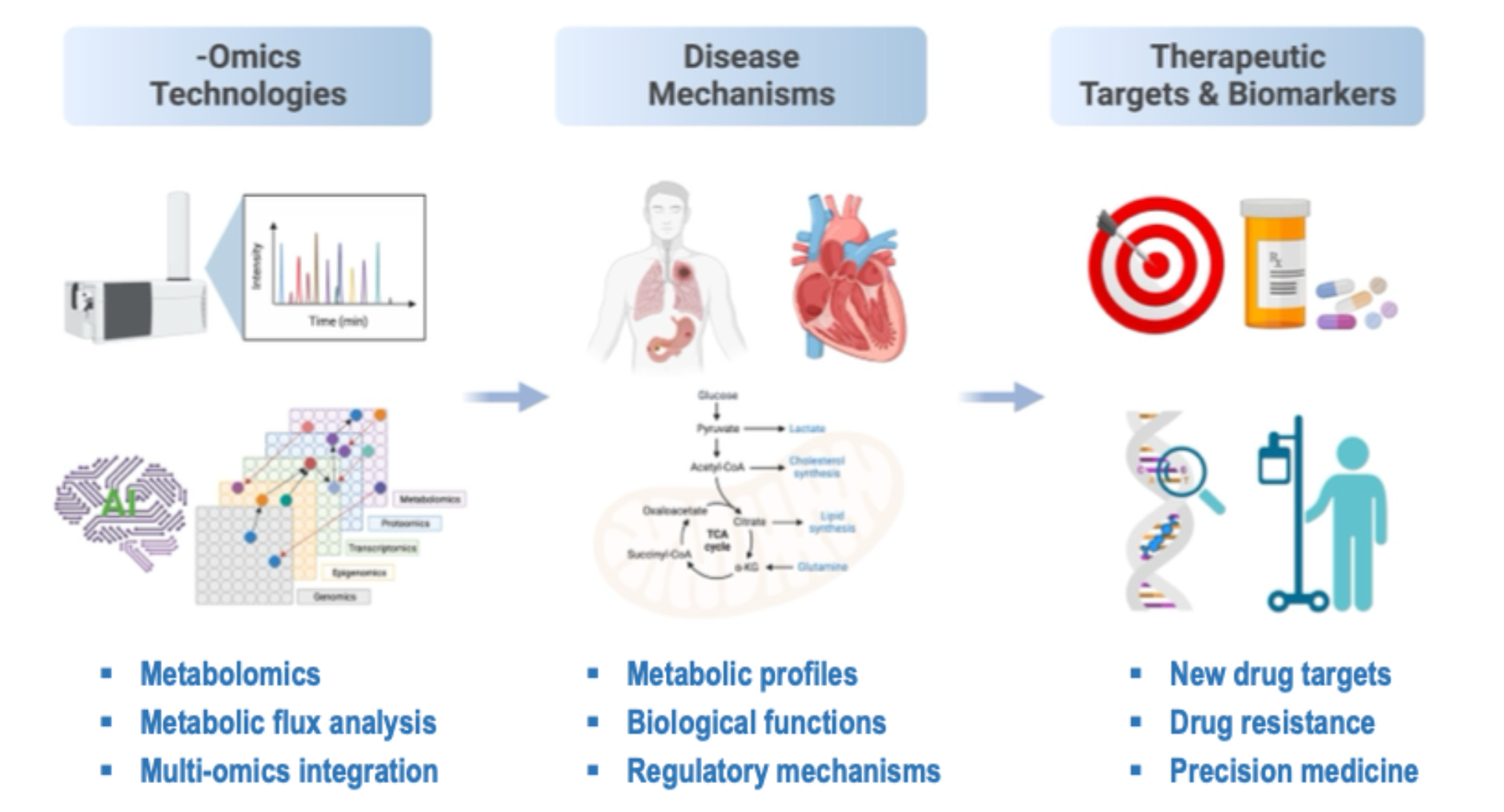
Research interests in Hu Lab
Research Highlights
1. Development of AI-Driven, Cross-Scale Metabolomics and Multi-Omics Platforms
We have developed high-sensitivity mass spectrometry–based platforms capable of profiling metabolic dynamics in ultra-low-input biological samples, including early-stage embryos and small cell populations. By integrating innovative chemical derivatization strategies, metabolic flux tracing, and AI-powered data analysis pipelines, these technologies enable precise, quantitative investigation of metabolic regulation in developmental biology, stem cell systems, and disease models.
2. Dissecting Metabolic Reprogramming in Cancer and Other Major Diseases
Our research systematically elucidates metabolic rewiring and intercellular metabolic interactions in cancer (e.g., lung, pancreatic, breast) and cardiovascular diseases. These studies have uncovered clinically relevant metabolic subtypes, resistance-associated metabolic circuits, and actionable pathways involving purine, acetylcholine, and bile acid metabolism. We have also extended this framework to viral diseases, revealing metabolic determinants of host–pathogen interactions through multi-omics–guided metabolic pathology.
3. Drug Target Discovery and Translational Applications
Leveraging integrative metabolomics and AI-assisted multi-omics analytics, we have contributed to the identification of therapeutic targets, molecular subtypes, and diagnostic biomarkers across multiple disease models, including early-stage lung adenocarcinoma, gastric cancer, and hypertrophic cardiomyopathy. Several studies have highlighted targetable metabolic pathways and drug repurposing opportunities, establishing a translational research pipeline that connects mechanistic discovery with functional validation and drug development.
Honors & Awards
- 2017, Bayer Investigator Award
- 2019, Second Prize, 14th National Forum on Youth in Medicine and Health
- 2019, Outstanding Member, Cancer Metabolism Branch, Chinese Anti-Cancer Association
- 2019, Excellent Teaching Award, Tsinghua University
- 2020, Bayer Investigator Award
- 2022, Excellent Ph.D. Dissertation Advisor, Tsinghua University
- 2022, Distinguished Professor, “Changjiang Scholars Program”
Selected Publications
(*Corresponding author; #First author; in reverse chronological order)
Research articles
1. Chen Y#, Wang B#, Zhao Y#, Shao X#, Wang M#, Ma F#, Yang L, Nie M, Jin P, Yao K, Song H, Lou S, Wang H, Yang T, Tian Y*, Han P*, Hu ZP*. Metabolomics machine learning predictor of diagnosis and prognosis of gastric cancer. Nature Communications. 2024;15(1),1657.
2. Fan H#, Xu Z#, Yao K#, Zheng B, Zhang Y, Wang X, Zhang T, Li X, Hu H, Yue B*, Hu ZP*, Zheng H*. Osteoclast cancer cell metabolic cross-talk confers PARP inhibitor resistance in bone metastatic breast cancer. Cancer Research. 2024;84(3),449-467.
3. Nie M#, Chen N#, Pang H#, Jiang T#, Jiang W, Tian P, Yao L, Chen Y, DeBerardinis RJ, Li W, Yu Q, Zhou C, Hu ZP*. Targeting acetylcholine signaling modulates persistent drug tolerance in EGFR-mutant lung cancer and impedes tumor relapse. Journal of Clinical Investigation. 2022;132(20),e160152.
4. Yuan M#, Tu B#, Li H#, Pang H#, Zhang N, Bai J, Shu Z, Christopher C, Huo S, Zhai J, Yao K, Wang L, Ying H, Zhu WG, Fu D, Hu ZP*, Zhao Y*. Cancer associated fibroblasts employ NUFIP1-dependent autophagy to secrete nucleosides and support pancreatic tumor growth. Nature Cancer. 2022;3(8),945-960.
5. Wang W#, Wang J#,*, Yao K#, Wang S#, Nie M, Xu J, Wu G, Lu M, Pei H, Luo X, Li D, Yang T, Li P, Song L*, Hu ZP*. Metabolic characterization of hypertrophic cardiomyopathy in human hearts. Nature Cardiovascular Research. 2022;1,445–461.
6. Jiang Y*,#, Deng Y#, Pang H#, Ma T, Ye Q, Chen Q, Chen H, Hu ZP*, Qin CF*, Xu Z*. Treatment of SARS-CoV-2 induced pneumonia with NAD+ and NMN in two mouse models. Cell Discovery. 2022;8(1),38.
7. Cheng W#, Pang H#, Campen MJ, Zhang J, Li Y, Gao J, Ren D, Ji X, Rothman N, Lan Q, Zheng Y, Leng S*, Hu ZP*, Tang J*. Circulatory metabolites trigger ex vivo arterial endothelial cell dysfunction in population chronically. Particle and Fibre Toxicology. 2022;19(1),20.
8. Nie M#, Yao K#, Zhu X#, Chen N, Xiao N, Wang Y, Peng B, Yao LA, Li P, Zhang P*, Hu ZP*. Evolutionary metabolic landscape from preneoplasia to invasive lung adenocarcinoma with therapeutic implications. Nature Communications. 2021;12(1),6479.
9. Zhao J#, Yao K#, Yu H#, Zhang L#, Xu Y#, Chen L, Sun Z, Zhu Y, Zhang C, Qian Y, Ji S, Pang H, Zhang M, Chen J, Correia C, Weiskittel T, Lin DW, Zhao Y, Chandrasekaran S, Fu X, Zhang D, Fan HY, Xie W, Li H, Hu ZP*, Zhang J*. Metabolic remodeling during early murine embryo development. Nature Metabolism. 2021;3(10),1372-1384.
10. Pang H#, Jiang Y#, Li J#, Wang Y#, Nie M#, Xiao N, Wang S, Song Z, Ji F, Chang Y, Zheng Y, Yao K, Yao L, Li S, Song L*, Lan X*, Xu Z*, Hu ZP*. Aberrant NAD+ metabolism underlies ZIKA virus-induced microcephaly. Nature Metabolism. 2021;3(8),1109-1124.
11. Xiao N#, Nie M#, Pang H#, Wang B#, Hu J#, Meng X, Li K, Ran X, Long Q, Deng H, Chen N, Li S, Tang N*, Huang A*, Hu ZP*. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nature Communications. 2021;12(1),1618.
12. Meng X#, Pang H#, Sun F, Jin X, Wang B, Yao K, Yao L, Wang L, Hu ZP*. Simultaneous 3-nitrophenylhydrazine derivatization strategy of carbonyl, carboxyl and phosphoryl submetabolome for LC-MS/MS-based targeted metabolomics with improved sensitivity and coverage. Analytical Chemistry. 2021;93(29),10075-10083.
13. Shi Z#, Xu S#, Xing S, Yao K, Zhang L, Xue L, Zhou P, Wang M, Yan G, Yang P, Liu J, Hu ZP*, Lan F*. Mettl17, a regulator of mitochondrial ribosomal RNA modifications, is required for the translation of mitochondrial coding genes. The FASEB Journal. 2019;33(11),13040-13050.
14. Huang F, Ni M, Chalishazar MD, Huffman KE, Kim J, Cai L, Shi X, Cai F, Zacharias LG, Ireland AS, Li K, Gu W, Kaushik AK, Liu X, Gazdar AF, Oliver TG, Minna JD, Hu ZP*, DeBerardinis RJ*. Inosine monophosphate dehydrogenase dependence in a subset of small cell lung cancers. Cell Metabolism. 2018;28(3),369-382.
15. Li XK#, Lu QB#, Chen WW#, Xu W#, Liu R, Zhang SF, Du J, Li H, Yao K, Zhai D, Zhang PH, Xing B, Cui N, Yang ZD, Yuan C, Zhang XA, Xu Z, Cao WC*, Hu ZP*, Liu W*. Arginine deficiency is involved in thrombocytopenia and immunosuppression in Severe Fever with Thrombocytopenia Syndrome. Science Translational Medicine. 2018;10(459), eaat4162.
Invited reviews
16. Xu Y, Jiang X, Hu ZP*. Synergizing metabolomics and artificial intelligence for advancing precision oncology. Trends in Molecular Medicine. 2025,S1471-4914(25)00016-4.
17. Nie M*, Hu ZP*. Metabolic orchestration of drug-tolerant persister cells in cancer. Life Medicine. 2024;3(6),lnae040.
18. Wang F#, Fu K#, Wang Y, Pan C, Wang X, Liu Z, Yang C, Zheng Y, Li X, Lu Y, To KKW, Xia C, Zhang J, Shi Z, Hu ZP*, Huang M*, Fu L*. Small-molecule agents for cancer immunotherapy. Acta Pharmaceutica Sinica B (APSB). 2024;14(3),905-952.
19. Thompson CB*, Vousden KH*, Johnson RS*, Koppenol WH*, Sies H*, Lu Z*, Finley LWS*, Frezza C*, Kim J*, Hu ZP*, Bartman CR*. A century of the Warburg effect. Nature Metabolism. 2023;5(11),1840-1843.
20. Pang H, Hu ZP*. Metabolomics in drug research and development, advances in technologies and applications. Acta Pharmaceutica Sinica B (APSB). 2023;13(8),3238-3251.
21. Wang B#, Yao K#, Hu ZP*. Advances in mass spectrometry-based single-cell metabolite analysis. TrAC Trends in Analytical Chemistry. 2023;163,117075.
22. Liang L, Sun F, Wang H, Hu ZP*. Metabolomics, metabolic flux analysis and cancer pharmacology. Pharmacology & Therapeutics. 2021;224,107827.
23. Pang H, Jia W*, Hu ZP*. Emerging applications of metabolomics in clinical pharmacology. Clinical Pharmacology & Therapeutics. 2019;106(3),544-556.
Co-authored papers
24. Hu Y, Yang Y, Tan P, Zhang Y, Han M, Yu J, Zhang X, Jia Z, Wang D, Yao K, Pang H, Hu ZP, Li Y, Ma T, Liu K, Ding S. Induction of mouse totipotent stem cells by a defined chemical cocktail. Nature. 2023;617(7962),792-797.
25. Agathocleous M, Meacham C, Burgess RJ, Piskounova E, Bruner E, Cowin B, Crane GM, Murphy MM, Hu ZP, DeBerardinis RJ, Morrison SJ. Ascorbate regulates haematopoietic stem cell function and suppresses leukaemogenesis. Nature. 2017;549(7673),476-81.
26. Kim J, Hu ZP, Cai L, Choi E, Rodriguez-Canales J, Villalobos P, Lin YF, Ni M, Unsal-Kacmaz K, Peña CG, Castrillon DH, Chen BPC, Wistuba I, Minna JD, DeBerardinis RJ. CPS1 maintains pyrimidine pools and DNA synthesis in KRAS/LKB1-mutant lung cancer cells. Nature. 2017;546(7656),168-72.
27. Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah A, Zhang H, Faber JE, Kinter MT, Szweda LI, Xing C, Hu ZP, Deberardinis RJ, Schiattarella G, Hill JA, Oz O, Lu Z, Zhang CC, Kimura W, Sadek HA. Hypoxia induces heart regeneration in adult mice. Nature. 2017;541(7636),222-7.
28. Piskounova E, Agathocleous M, Murphy MM, Hu ZP, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ, Morrison SJ. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527(7577),186-91.
29. DeNicola GM, Chen PH, Mullarky E, Sudderth JA, Hu ZP, Wu D, Tang H, Xie Y, Asara JM, Huffman KE, Wistuba II, Minna JD, DeBerardinis RJ, Cantley LC. NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nature Genetics. 2015;47(12),1475-81.
30. Srivastava N, Kollipara RK, Singh DK, Sudderth J, Hu ZP, Nguyen H, Wang S, Humphries CG, Carstens R, Huffman KE, DeBerardinis RJ, Kittler R. Inhibition of cancer cell proliferation by PPARγ is mediated by a metabolic switch that increases reactive oxygen species levels. Cell Metabolism. 2014;20(4),650-61.
31. Curtis MM, Hu ZP, Klimko C, Narayanan S, Deberardinis R, Sperandio V. The gut commensal Bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape. Cell Host & Microbe. 2014;16(6),759-69.
32. Mullen AR, Hu ZP, Shi X, Jiang L, Boroughs LK, Kovacs Z, Boriack R, Rakheja D, Sullivan LB, Linehan WM, Chandel NS, DeBerardinis RJ. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Reports. 2014;7(5),1679-90.






