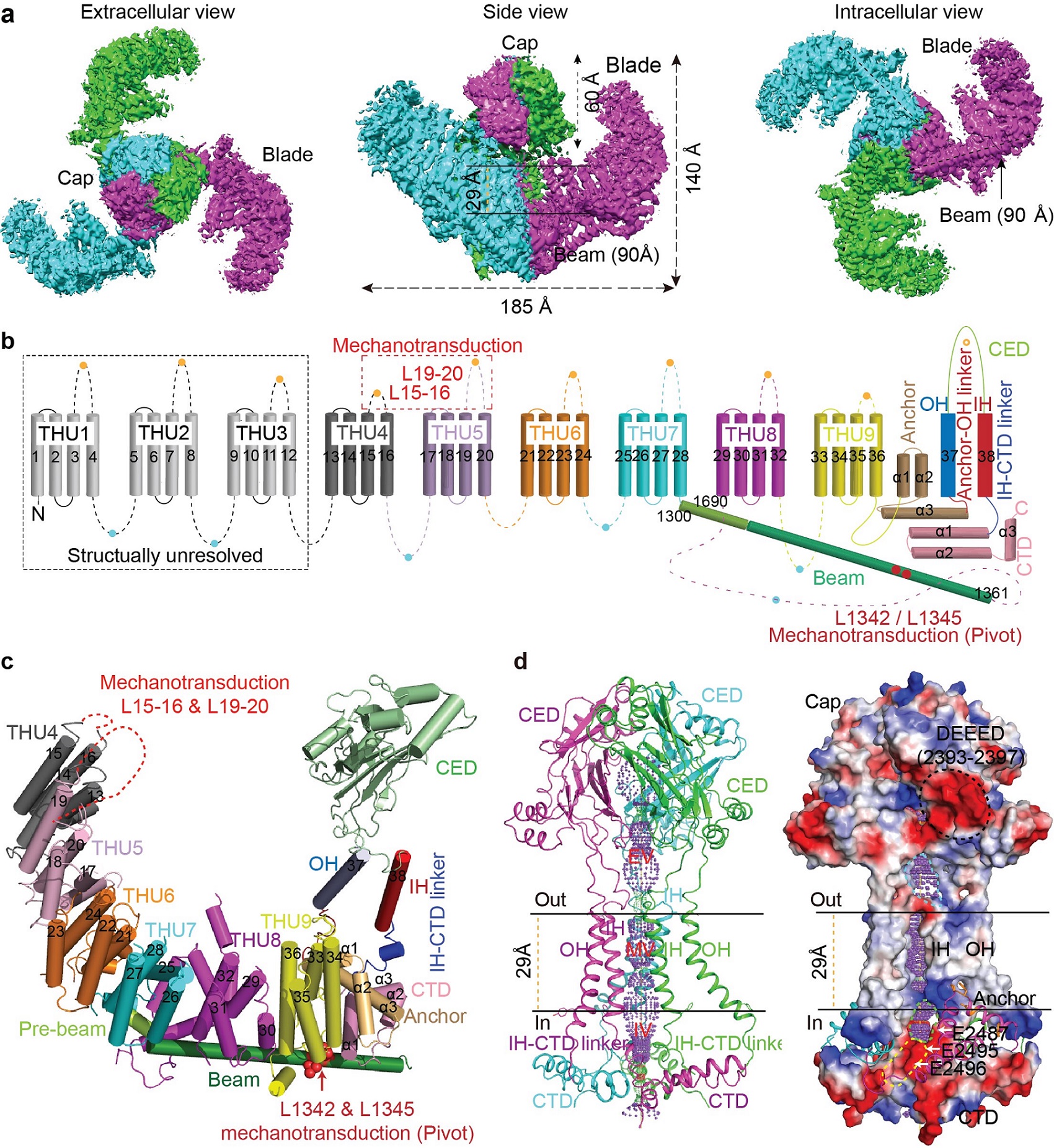
The mechanosensitive Piezo channels function as key eukaryotic mechanotransducers. However, their structures and mechanogating mechanisms remain unknown. Here, we determine the three-bladed, propeller-like cryo-EM structures of mouse Piezo1 and functionally reveal its mechanotransduction components. Despite the lack of sequence repetition, we identify 9 repetitive units constituted of 4 transmembrane (TM) helices each, termed THUs, assembling into the highly curved blade-like structure. The last TM encloses a hydrophobic pore, followed by three intracellular fenestration sites and side portals comprising pore-property-determining residues. The central region forms a 90 Å-long intracellular beam-like structure, which undergoes a lever-like motion for connecting THUs to the pore via the interfaces of the C-terminal domain, anchor-resembling domain and outer helix. Deleting extracellular loops in the distal THUs or mutating single residues in the beam impairs the mechanical activation of Piezo1. Overall, Piezo1 possesses an unprecedented 38-TM topology and designated mechanotransduction components, enabling a lever-like mechanogating mechanism.