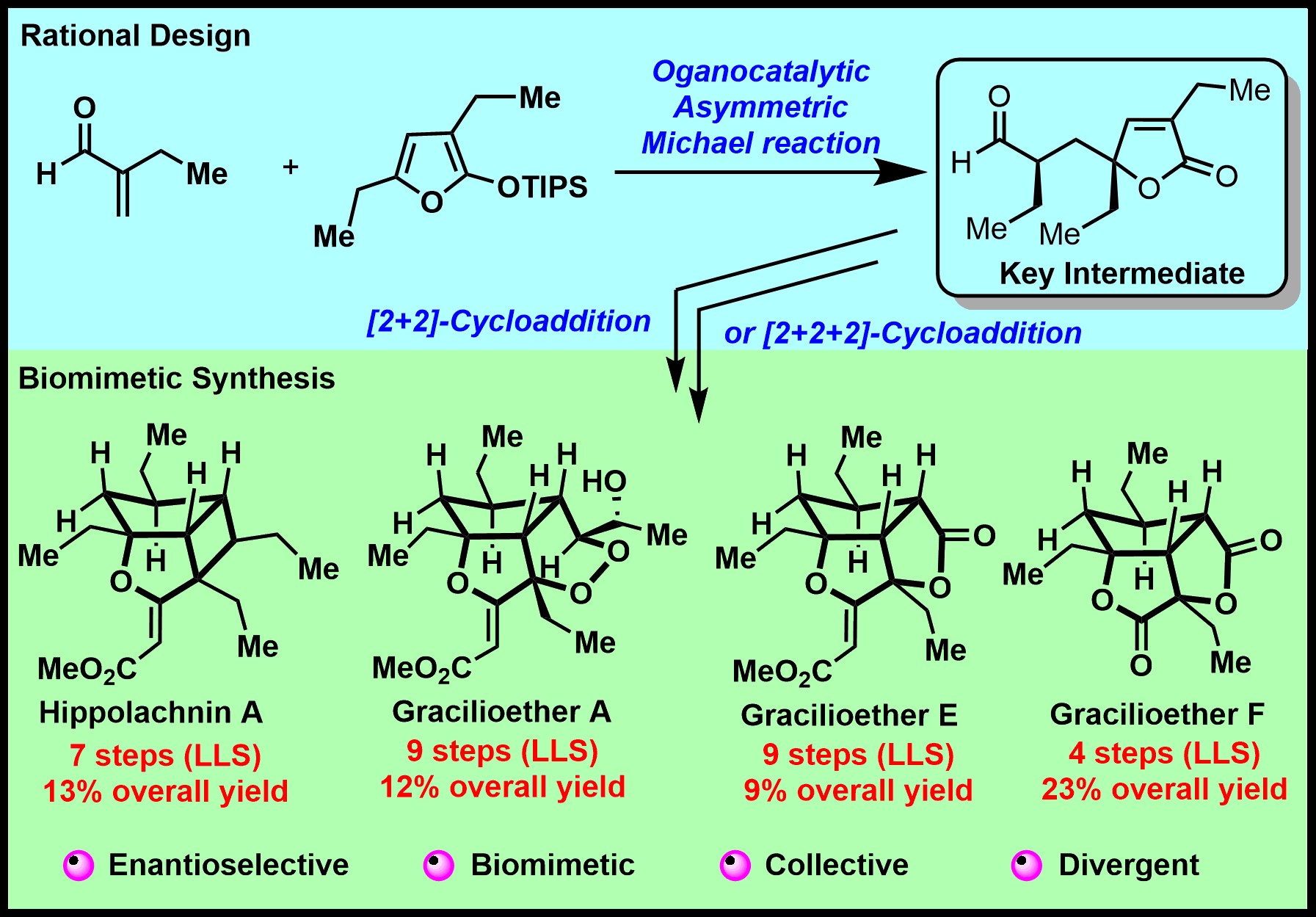
The Plakortin polyketides represent a structurally and biologically fascinating class of marine natural products. Herein, we report a unified strategy that enables the divergent syntheses of various Plakortin polyketides with high step-economy and overall efficiency. As proof-of-concept cases, the enantioselective total syntheses of (+)-hippolachnin A, (+)-gracilioether A, (−)-gracilioether E, and (−)-gracilioether F have been accomplished based on a series of bio-inspired, rationally designed, or serendipitously discovered transformations, which include (1) an organocatalytic asymmetric 1,4-conjugate addition to assemble the common chiral γ-butenolide intermediate enroute to all of the aforementioned targets, (2) a challenging biomimetic [2+2] photocycloaddition to forge the oxacyclobutapentalene core of (+)-hippolachnin A, (3) a [2+2] photocycloaddition followed by one-pot oxidative cleavage of methyl ether/Baeyer–Villiger rearrangement to access (−)-gracilioether F, and (4) an unprecedented hydrogen-atom-transfer-triggered oxygenation of vinylcyclobutane to afford (+)-gracilioether A and (−)-gracilioether E in one pot.