Research Interests:
1. Intestinal stem cell research: We focus on the study of patient-derived intestinal stem cells in ulcerative colitis, and the impact of cytokines or microbial metabolites in the pathological microenvironment on intestinal stem cells. We also investigate the aging of intestinal stem cells.
2. Liver-related research: We focus on the pathological changes in liver stem cells/cholangiocytes during liver fibrosis and their role in disease onset. Our work also includes clinical translational research on liver stem cell transplantation.
3. Tumor-related research: We are dedicated to translational research in precision medicine utilizing patient-derived 2D cancer cell lines and 3D air-liquid interface (ALI) organoid technology systems. Our research primarily involves the development and refinement of strategies for predicting primary tumor metastatic organs and assessing the risk of primary tumor metastasis.
4. Drug absorption models: We also study the translational application of gastric and intestinal air-liquid interface organoid models in drug absorption.
Scientific Contributions:
Prof. Wang has been working on adult stem cells and human diseases. She and her colleagues discovered that a discrete population of cells at the junction of the esophagus and stomach is the cellular origin of Barrett’s metaplasia. This novel mechanism underlying the etiology of Barrett’s Esophagus transformed people’s review in early diagnosis and preventive therapy targeting cancer precursors of esophageal cancer. This finding was published in Cell as a featured article. Additionally, Prof. Wang has established a technology platform in cloning ground-state epithelial stem cells that can be propagated robustly and faithfully in vitro. It has been demonstrated that these stem cells, after long-term culture, still have the long-term self-renewal capacity and could maintain precise lineage commitment. Moreover, she also established a 3D culture system that can precisely recapitulate intestinal epithelium architecture in vitro and showed that this system can be applied to model intestinal diseases, such as pseudomembranous colitis (Wang X. et al., Nature, 2015).
Dr. Xia Wang’s group has developed an efficient and stable patient-derived 2D cancer cell line and 3D air-liquid interface (ALI) organoid technology platform (Zhao et al., Advanced Science, 2022). This platform combines the advantages of 2D cancer cell lines and 3D tumor organoids, which not only accurately recapitulate the characteristics of primary tumors, but also successfully captures the inter- and intra-tumor heterogeneity in drug responses and tumorigenicity. This platform can be used to build a tumor organoid biobank for drug screening, offering personalized drug sensitivity predictions for cancer patients and helping to advance personalized and precision medicine. Using this platform, Dr. Xia Wang's group and her collaborative team successfully established single-cell derived cancer cell lines origin from primary tumor tissues of colorectal cancer patients. They identified metastatic seeds in primary colorectal cancer tissues, comprehensively and systematically described their cellular and molecular characteristics, and revealed the molecular mechanism of their organ-specific metastasis. They also identified a set of marker genes that can predict and assess metastasis risk in primary colorectal cancer tumors (Zhao et al., Journal of Experimental Medicine, 2022). This research not only paves the way for studying tumor metastasis mechanisms but also provides new strategies and directions for future cancer treatments.
Selected Achievements
1. An 2D intestinal stem cell and 3D ALI organoid culture system
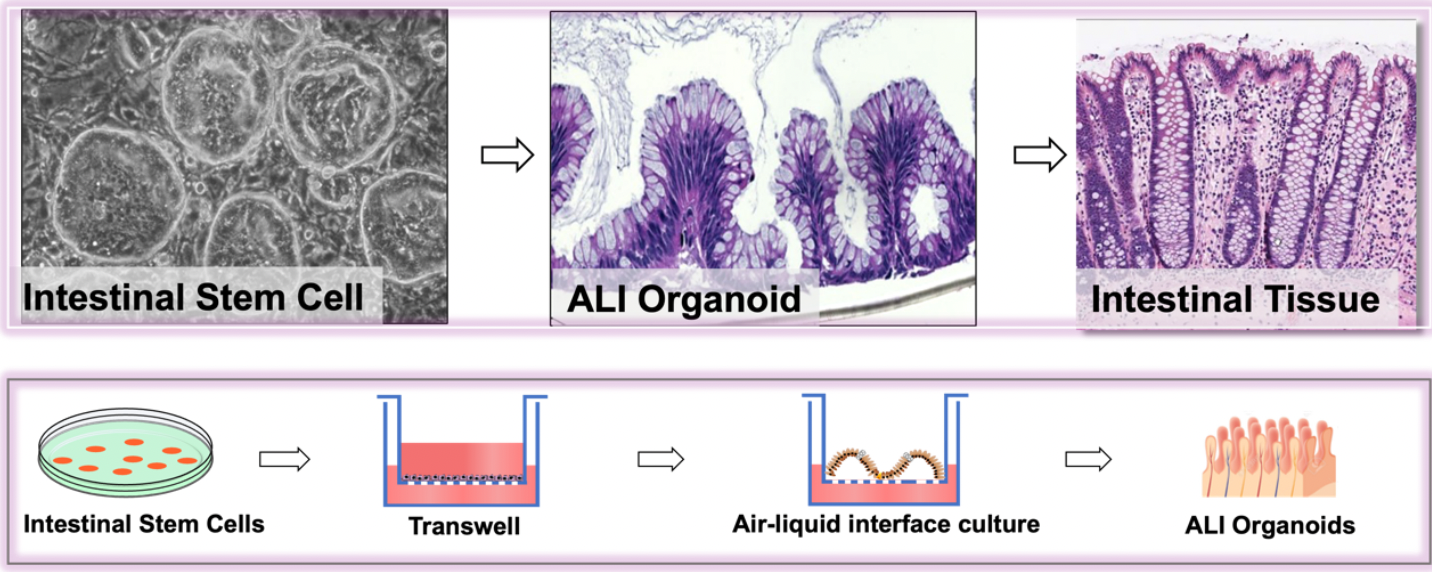
Figure 1. Schematic diagram of intestinal stem cell ALI organoid technology system (Wang et al., Nature, 2015).
2. An patient-derived 2D cancer cell line and 3D ALI tumor organoid technology platform
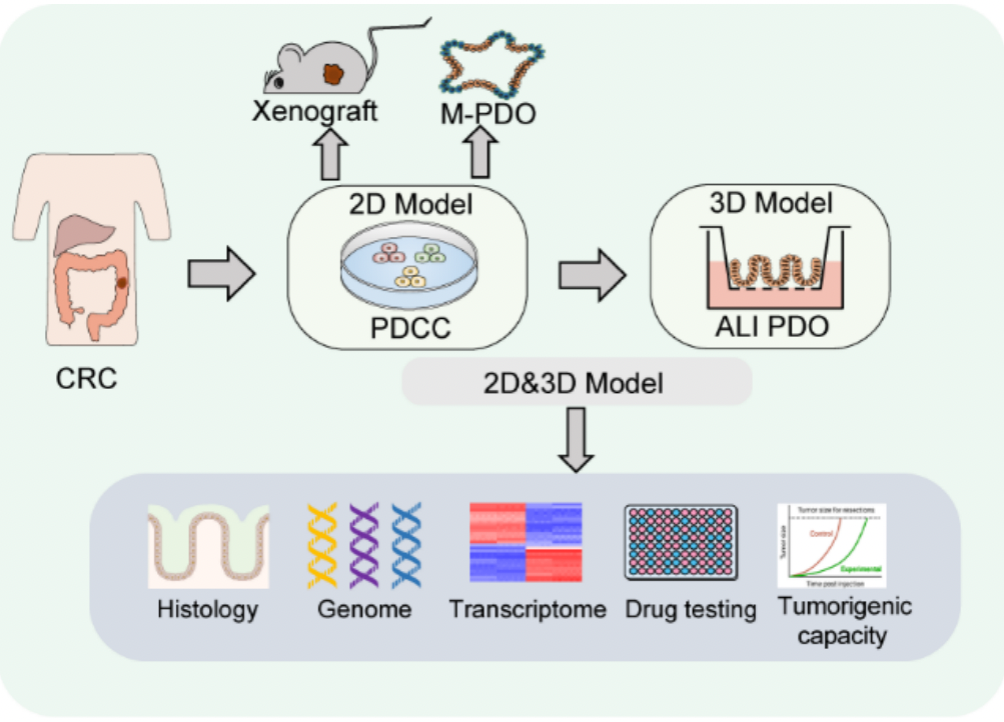
Figure 2. Schematic diagram of 2D cancer cell line and 3D ALI tumor organoid technology platforms(Zhao et al., Advanced Science, 2022).
3. Metastatic seeds in primary tumors, a new target for precision medicine
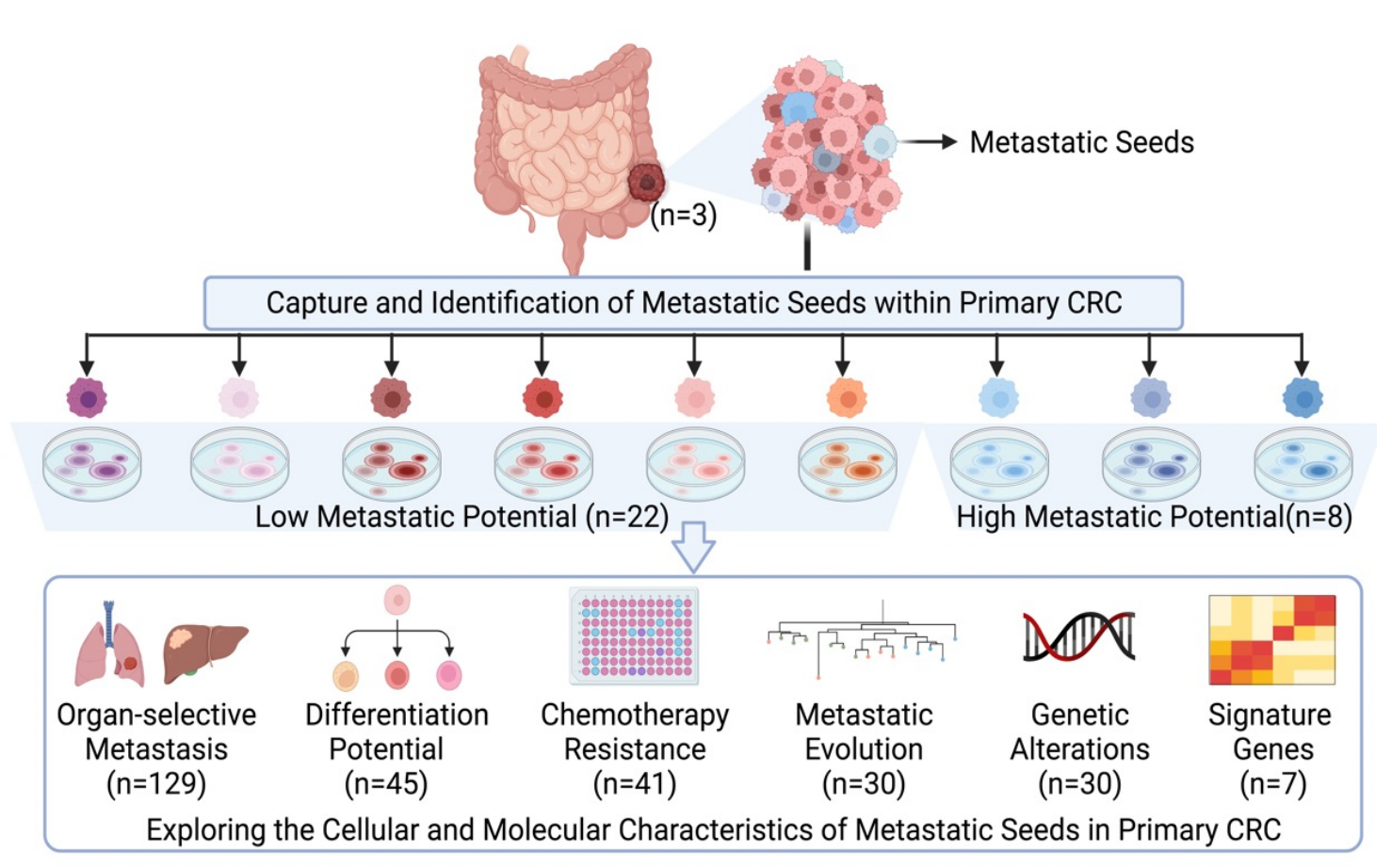
Figure 3. Systematic description of metastatic seeds in primary colorectal cancer (Zhao et al., Journal of Experimental Medicine, 2024).
Honors and Awards
Bayer Investigator (2018)
Bayer Investigator (2017)
The Thousand Talents Plan (Youth) (2016)
Harvard Chinese Life Science Annual Distinguished Research Award (2012)
Zhu Liyuehua Outstanding Doctoral Award of Chinese Academy of Sciences (2009)
Outstanding graduate student of Institute of Genetics and Developmental Biology Chinese Academy of Sciences (2009)
Outstanding Thesis of Institute of Genetics and Developmental Biology Chinese Academy of Sciences (2009)
President Scholarship of Chinese Academy of Sciences (2005)
Selected Publications:
1. Yuanyuan Zhao#, Bing Zhang#, Yiming Ma#, Mengmeng Guo, Fuqiang Zhao, Jianan Chen, Bingzhi Wang, Hua Jin, Fulai Zhou, Jiawei Guan, Qian Zhao, Qian Liu*, Hongying Wang*, Fangqing Zhao*, Xia Wang*. Distinct molecular profiles drive multifaceted characteristics of colorectal cancer metastatic seeds. Journal of Experimental Medicine (2024 May 6;221(5):e20231359. doi: 10.1084/jem.20231359.)
2. Chuandong Liu#, Jie Li#, Hua Jin#, Qian Zhao#, Fangle Li, Zurui Huang, Boyuan Mei, Wenxuan Gong*, Xia Wang*, Dali Han*. Colonic stem cell from severe ulcerative colitis maintains environment-independent immune activation by altering chromatin accessibility and global m 6A loss. Life Medicine, 2023. 2 (4):1-14.
3. Yuanyuan Zhao#, Mengmeng Guo#, Fuqiang Zhao, Qian Liu and Xia Wang*. Colonic stem cells from normal tissues adjacent to tumor drive inflammation and fibrosis in colorectal cancer. Cell Communication and Signaling, 2023, 21:186.
4. Aging Biomarker Consortium, Hainan Bao#, Jiani Cao#, Mengting Chen#, Min Chen#, Wei Chen#, Xiao Chen#, Yanhao Chen#, Yu Chen#, Yutian Chen#, Zhiyang Chen#, Jagadish K. Chhetri#, Yingjie Ding#, Junlin Feng#, Jun Guo#, Mengmeng Guo#, Chuting He#, Yujuan Jia#, Haiping Jiang#, Ying Jing#, Dingfeng Li#, Jiaming Li#, Jingyi Li#, Qinhao Liang#, Rui Liang#, Feng Liu#, Xiaoqian Liu#, Zuojun Liu#, Oscar Junhong Luo#, Jianwei Lv#, Jingyi Ma#, Kehang Mao#, Jiawei Nie#, Xinhua Qiao#, Xinpei Sun#, Xiaoqiang Tang#, Jianfang Wang#, Qiaoran Wang#, Siyuan Wang#, Xuan Wang#, Yaning Wang#, Yuhan Wang#, Rimo Wu#, Kai Xia#, Fu-Hui Xiao#, Lingyan Xu#, Yingying Xu#, Haoteng Yan#, Liang Yang#, Ruici Yang#, Yuanxin Yang#, Yilin Ying#, Le Zhang#, Weiwei Zhang#, Wenwan Zhang#, Xing Zhang#, Zhuo Zhang#, Min Zhou#, Rui Zhou#, Qingchen Zhu#, Zhengmao Zhu#, Feng Cao*, Zhongwei Cao*, Piu Chan*, Chang Chen*, Guobing Chen*, Hou-Zao Chen*, Jun Chen*, Weimin Ci*, Bi-Sen Ding*, Qiurong Ding*, Feng Gao*, Jing-Dong J. Han*, Kai Huang*, Zhenyu Ju*, Qing-Peng Kong*, Ji Li*, Jian Li*, Xin Li*, Baohua Liu*, Feng Liu*, Lin Liu*, Qiang Liu*, Qiang Liu*, Xingguo Liu*, Yong Liu*, Xianghang Luo*, Shuai Ma*, Xinran Ma*, Zhiyong Mao*, Jing Nie*, Yaojin Peng*, Jing Qu*, Jie Ren*, Ruibao Ren*, Moshi Song*, Zhou Songyang*, Yi Eve Sun*, Yu Sun*, Mei Tian*, Shusen Wang*, Si Wang*, Xia Wang*, Xiaoning Wang*, Yan-Jiang Wang*, Yunfang Wang*, Catherine C. L. Wong*, Andy Peng Xiang*, Yichuan Xiao*, Zhengwei Xie*, Daichao Xu*, Jing Ye*, Rui Yue*, Cuntai Zhang*, Hongbo Zhang*, Liang Zhang*, Weiqi Zhang*, Yong Zhang*, Yun-Wu Zhang*, Zhuohua Zhang*, Tongbiao Zhao*, Yuzheng Zhao*, Dahai Zhu*, Weiguo Zou*, Gang Pei* & Guang-Hui Liu*. Biomarkers of aging. Science China Life Sciences. 2023. 66: 893-1066.
5. Yuanyuan Zhao#, Bing Zhang#, Yiming Ma, Fuqiang Zhao, Jianan Chen, Bingzhi Wang, Hua Jin, Fulai Zhou, Jiawei Guan, Qian Zhao, Hongying Wang, Qian Liu*, Fangqing Zhao*, Xia Wang*. Colorectal cancer patient-derived 2D and 3D models efficiently recapitulate inter- and intra-tumoral heterogeneity. Advanced Science. 2022 (DOI: 10.1002/advs.202201539)
6. Qian Zhao#, Jiawei Guan, Xia Wang*. Intestinal stem cells and intestinal organoids. Journal of Genetics and Genomics. 2020. 47(6):289-299. (Review)
7. Yamamoto Y*, Wang X*(*Co-first authors), Bertrand D, Kern F, Zhang T, Deluba M, Srivastava S, Khor CC, Hu YY, Wilson L, Blaszyk H, Rolshud D, Ming T,Liu JJ, Howitt B, Vincent M, Crum CP, Nagarajan N, Ho KY, McKeon F, and Xian W. Mutational spectrum of Barrett’s stem cells suggests paths to initiation of a precancerous lesion. Nature Communications. 2016. doi: 10.1038/ncomms10380.
8. Wang X*, Yamamoto Y*(*Co-first authors), Wilson LH, Zhang T, Howitt B, Ning G, Hong Y, Khor CC, Chevalier B, Bertrand D, Nagarajan N, Sylvester FA, Farrow MA, Lacy DB, Ho KY, Crum CP, McKeon F and Xian W. Cloning and variation of ground state intestinal stem cells. Nature. 2015. 522(7555):173-178.
9. Wang X*, Ouyang H*, Yamamoto Y*, (*Co-first authors), Kumar PA, Wei TS, Dagher R, Vincent M, Lu X, Bellizzi AM, Ho KY, Crum CP, Xian W, McKeon F. Residual embryonic cells as precursors of a Barrett's-like metaplasia. Cell. 2011. 145(7):1023-35. Featured Article in Cell. Highlighted in Nature Reviews Cancer. Faculty 1000 Biology: 10 (Exceptional).
10. Wang, X and Dai, J. Isoforms of OCT4 contribute to the confusing diversity in stem cell biology. Stem Cells. 2010. 28(5):885-93. (Invited Review)
11. Gao, Y., Wang, X. *(* Co-first authors), Han, J., Xiao, Z., Chen, B., Su, G., Dai, J. The novel OCT4 spliced variant OCT4B1 can generate three protein isoforms by alternative splicing into OCT4B. J Genet Genomics. 2010. 37(7):461-5
12. Zhang, J., Wang, X. *(* Co-first authors), Chen, B., Xiao, Z., Li, W., Lu, Y. and Dai, J. The human pluripotency gene NANOG/NANOGP8 is expressed in gastric cancer and associated with tumor development. Oncology Letters. 2010. 1(3):457-463.
13. Wang, X., Zhao, Y., Xiao, Z., Chen, B., Wei, Z., Wang, B., Zhang, J., Han, J., Gao, Y., Li, L., Zhao, H., Zhao, W., Lin, H. and Dai, J. Alternative translation of OCT4 by an internal ribosome entry site and its novel function in stress response. Stem Cells. 2009. 27(6):1265-75.
14. Zhang, J.*, Wang, X.* (* Co-first authors), Han, J., Chen, B., Wang, B., Suo, G. and Dai J. NANOGP8 is a retrogene expressed in cancers. FEBS Journal. 2006. 273(8): 1723-30.



