Research Interests
Research interests of Prof. Rao's laboratory have been mainly focused on early drug discovery of anti-cancer and anti-infectious disease fields; developing new heterocycle chemistry which is the core of modern medicinal chemistry. A high-quality small molecule library is the key for successful early drug discovery. We have been employing synthetic methods developed in our laboratory to build up highly diverse and novel heterocycle libraries (typical heterocycles include pyrazole, indazole, quinoline, quinolone, pyridine, pyrimidine, benzopyran, benzofuran, imidazole, etc). Through collaborations with my colleagues in school of medicine, we have been able to identify a few interesting hit molecules which are highly effective against tumor, bacterial and virus, etc. Besides optimizing drug-like property of lead compounds, we also employ these biologically active compounds as chemical probes to identify drug targets and elucidate molecular mechanism with our collaborators.
Scientific Contributions
Prof. Rao has been working in the field of synthetic chemistry and medicinal chemistry over the past decade. In recent years, his group has been actively working in the construction of new heterocyclic compound libraries through a new strategy combining C-H functionalizations with Diversity Oriented Synthesis. Besides methodology developments, his group is also interested in developing novel therapeutics for infectious disease and cancer treatment. His group has been utilizing the expertises in the fields of medicinal chemistry, synthetic chemistry and chemical biology to develop lead compounds for important drug targets and explore new potential therapeutic targets.
Selected Achievements
1
. Significance of C-H hydroxylation: we report the first example of Pd(II) catalyzed regioselective C-H oxygenation of these substrates and its convenient applications in oxygen-containing heterocycle synthesis and late-stage drug modification. Significance of C-H halogenation: we report the first example of Pd(II) catalyzed regio- and chemoselective chlorination of aryl compounds, and its broad utilities in drug modification and early drug discovery.
Figure 1:Representative SP2 C-H hydroxylation and SP2 C-H halogenation.
2.
Significance of C-H alkoxylation: This new method represents the first example of using cyclic hypervalent iodine oxidants in C(sp3)-H bond alkoxylation and its broad utilities in drug modification and early drug discovery.
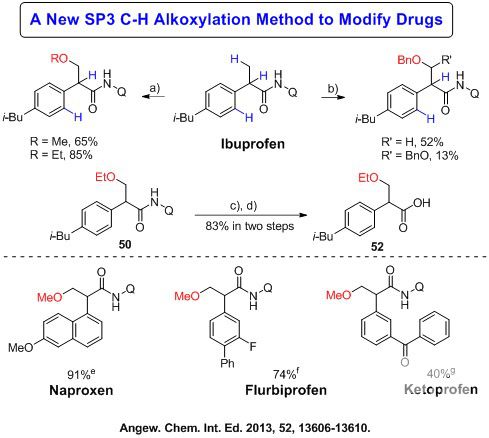
Figure 2:Representative SP2 C-H alkoxylation and application in drug modifications
3.
Significance of C-H Regio-selective SP2 C-H hydroxylation and application in drug synthesis: In this paper, we report the development of Pd(II)- or Ru(II)-catalyzed regioselective C-H hydroxylation of benzanilides, which provides a convenient method for the preparation of diverse
ortho-hydroxylated benzanilide compound library. This regioselective strategy as a means of accessing different types of hydroxylated benzanilides directly from benzanilide substrates is particularly attractive and depends highly on the practicality of such chemical processes.
Figure 3:Regio-selective SP2 C-H hydroxylation and application in drug synthesis.
Awards and Honors
2013 Supported by ‘221’ Research Program (Tsinghua University)
2013 Supported by Program for New Century Excellent Talents (Ministry of Education)
2013 Bayer young investigator awards
2012 Roche excellent research awards
Selected Publications
1.Y. Sun, T. Sun, Y. Wu, X. Zhang*, and
Y. Rao* ‘A Diversity-Oriented Synthesis of Bioactive Benzanilides by a Regioselective C(sp2)-H Hydroxylation Strategy’.
Chemical Science, 2016,
7, 2229-2238.
2.X. Sun, Y. Sun, C. Zhang, and
Yu Rao.* ’’ Room-Temperature Palladium-Catalyzed C-H Chlorination by Weak Coordination: One-Pot Synthesis of 2-Chlorophenols with Excellent Regioselectivity’’.
Chem. Commun., 2014,
50, 1262-1264.
3.G. Shan, X. Yang, Y. Zong and
Y. Rao.* "An Efficient Palladium Catalyzed C-H Alkoxylation of Unactivated Methylene and Methyl Groups with Cyclic Hypervalent Iodine (I
3+) Oxidants".
Angew. Chem. Int. Ed. 2013,
52, 13606.
4.X. Sun, G. Shan, Y. Sun, and
Y. Rao.* “A General Approach for Regio- and Chemoselective C-H Chlorination/Bromination of Electron-Deficient Arenes via Weak Coordination and A Preliminary Study of Priority Order of Relative Directing Group Ability”.
Angew. Chem. Int. Ed. 2013,
52, 4440-4444.
5.G. Shan, X. Yang, L. Ma, and
Y. Rao.* “Pd-Catalyzed C-H Oxygenation with TFA/TFAA: Expedient Access to Oxygen-Containing Heterocycles and Late-Stage Drug Modification”.
Angew. Chem. Int. Ed. 2012,
51, 13070-13074.



