Research Interests
Precisely modulating the immune system can potentially prevent or treat cancer, autoimmune diseases, and infectious diseases. Recent breakthroughs in discovering innovative therapeutic targets and molecules have created new opportunities to harness the immune system's power to treat different diseases. Still, the instability of therapeutic molecules and biological barriers at the tissue, cellular, and subcellular levels can prevent them from accessing the therapeutic targets and, therefore, compromise the therapeutic efficacy. Furthermore, the off-target effect can also cause severe side effects. My lab is interested in working at the interface of pharmaceutical sciences and immunotherapy and developing innovative formulations to overcome the above challenges and fully unleash the power of immunotherapy. The primary research areas include (1) developing innovative formulations that can overcome drug instability and biological barriers; (2) tuning the spatiotemporal distribution of drug molecules at different levels (e.g., tissue, cellular, and subcellular level) and investigating its relationship with the host immunity; (3) exploring the use of formulations for the treatment of different diseases (e.g., cancer, infectious diseases, and autoimmune diseases) and elucidating the mechanism of action.
Scientific Contributions
Dr. Rui Kuai has long been dedicated to interdisciplinary research in drug delivery systems and immunotherapy. He has (1) used bioinspired strategies to develop a series of lipid-based nanomedicines to overcome critical delivery barriers that limit the effectiveness of immunotherapy, (2)elucidated the key mechanisms by which these formulations regulate drug delivery efficiency at the tissue, cellular, and subcellular levels, (3) and established methods for utilizing these formulations to prevent and treat various diseases. Some of his early findings have led to the founding of a startup company named EVOQ Therapeutics.
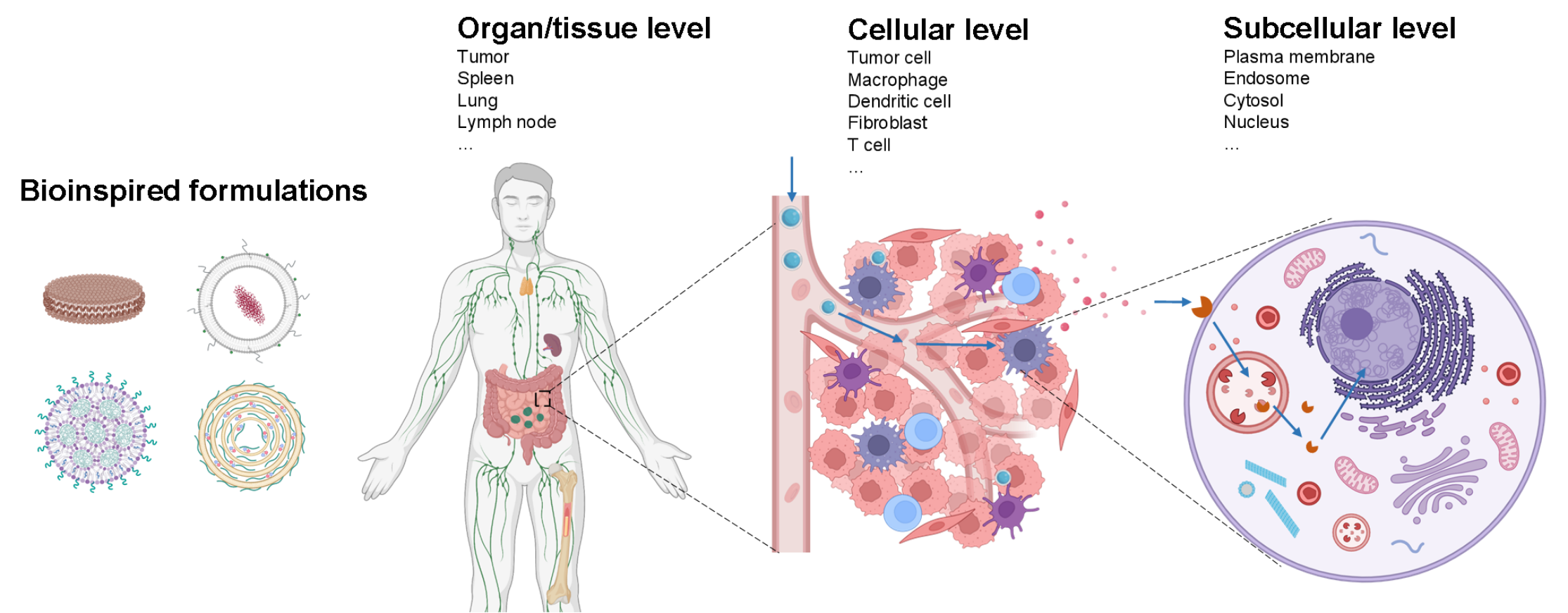
Selected Achievements
1.Lipid Nanomedicines Enhance Small Molecule-Triggered Immune Responses
Dr. Kuai has developed several lipid nanomedicines to address the delivery challenges of small-molecule drugs in activating antitumor immune responses. These include high-density lipoprotein (HDL)-mimetic lipid nanodiscs (Science Advances, 2018), ultrasound-responsive liposomes (Nature Communications, 2023), and "onion-like" multilamellar liposomes to improve the delivery efficiency of small molecules (ACS Nano, 2024). The studies elucidated the principles by which nanostructures and external stimuli influence the delivery behaviors of small-molecule drugs. Based on these insights, he has established effective methods to activate broad-spectrum antitumor immune responses for solid tumor treatment.
2.Lipid Nanovaccines for Precision Cancer Therapy
Dr. Kuai has developed several lipid nanovaccines to address tumor neoantigen peptides' delivery challenges in activating antitumor immune responses. These include high-density lipoprotein (HDL)-mimetic lipid nanodisc vaccines (Nature Materials, 2017) and ultrasound-responsive liposomal nanovaccines (Nature Communications, 2024), which enhance the delivery efficiency of peptide antigens to their targets. The research elucidated how factors such as the nanostructure of the formulations, the composition of the surface protein corona, and the fluidity of lipid membranes influence delivery behaviors. Based on these findings, he has established efficient methods for personalized cancer immunotherapy.
3.Lipid Nanomedicines Enhance Nucleic Acid-Triggered Immune Responses
Dr. Kuai has developed several lipid-based nanomedicines to address the delivery challenges of nucleic acids in activating antitumor immune responses. These include high-density lipoprotein (HDL)-mimetic lipid nanodiscs (Journal of Controlled Release, 2018) and virus-inspired liponanogels (Journal of Controlled Release, 2024), which improve the delivery efficiency of nucleic acids. The research elucidated the mechanisms by which nanostructures influence nucleic acid delivery. Based on these findings, he has established methods for efficiently activating mucosal immune responses to treat mucosal tumors (Cancer Research, 2024).
Honors and Awards
2024 First Prize in Tsinghua University Young Teacher’s Teaching Competition
2024 Excellent Teaching Award, School of Pharmaceutical Sciences, Tsinghua University
2021 The Science Fund Program for Distinguished Young Scholars
2018 Chinese Government Award for Outstanding Students Abroad
2017 American Association of Pharmaceutical Scientists Innovation in Biotechnology Award
2017 Norman Weiner Graduate Fellowship, University of Michigan
2015-2017 American Heart Association Predoctoral Fellowship
2013 Broomfield International Student Fellowship, University of Michigan
2013 Outstanding Master’s Thesis Award, Sichuan Province
2011 “China Shiyao Pharmaceutical Group Co., Ltd” Outstanding Student Award
Selected Publications
1. Yu J, Li X, Li J, Sun N, Peng C, Huang J, Li S,
Kuai R*. Single-dose physically crosslinked hyaluronic acid and lipid hybrid nanoparticles containing cyclic guanosine monophosphate–adenosine monophosphate eliminate established tumors.
ACS Nano. 2024;18(43):29942-29955.
2. Li X, Wu C, Li J, Yu J, Yang X, Yu L, Wang C,
Kuai R*. An immunostimulatory liponanogel reveals immune activation-enhanced drug delivery and therapeutic efficacy in cancer.
Journal of Controlled Release. 2024;376:167-183.
3. He J, Wang C, Fang X, Li J, Shen X, Zhang J, Peng C, Li H, Li S, Karp J,
Kuai R*. Tuning the fluidity and protein corona of ultrasound-responsive liposomal nanovaccines to program T cell immunity in mice.
Nature Communications. 2024 ;15(1):8121.
4. Li J, Luo L, He J, Yu J, Li X, Shen X, Zhang J, Li S, Karp JM,
Kuai R*. A Virus-Inspired Inhalable Liponanogel Induces Potent Antitumor Immunity and Regression in Metastatic Lung Tumors.
Cancer Research. 2024;84(14):2352-2363.
5. Li X#, Gao J#, Wu C, Wang C, Zhang R, He J, Xia ZJ, Joshi N*, Karp JM*,
Kuai R*. Precise modulation and use of reactive oxygen species for immunotherapy.
Science Advances. 2024;10(20):eadl0479.
6. Wang C, Zhang R, He J, Yu L, Li X, Zhang J, Li S, Zhang C, Kagan JC, Karp JM,
Kuai R*. Ultrasound-responsive low-dose doxorubicin liposomes trigger mitochondrial DNA release and activate cGAS-STING-mediated antitumour immunity.
Nature Communications. 2023;14(1):3877.
7. Levy O#,
Kuai R#, Siren EM#, Bhere D, Milton Y, Nissar N, Debiasia M, Heinelt M, Abdi R, Alturki M, Fallatah M, Almalik A, Alhansan AH, Shah K, Karp JM. Shattering the glass ceiling of clinically meaningful mesenchymal stromal cell (MSC) therapies.
Science Advances, 2020, 6 (30), eaba6884.
8.
Kuai R, Sun X, Yuan W, Ochyl LJ, Xu Y, Najafabadi AH, Scheetz L, Yu M, Balwani I, Schwendeman A*, Moon JJ*. Dual TLR agonist nanodiscs as a strong adjuvant system for vaccines and immunotherapy.
Journal of Controlled Release, 2018, 282, 131-139.
9.
Kuai R#, Yuan W#, Son S, Nam J, Xu Y, Fan Y, Schwendeman A*, Moon JJ*. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy.
Science Advances. 2018; 4: eaao1736.
10.
Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A*, Moon JJ*. Designer vaccine nanodiscs for personalized cancer immunotherapy.
Nature Materials. 2017, 16 (4), 489-496.
11.
Kuai R, Li D, Chen YE, Moon JJ*, Schwendeman A*. High-density lipoproteins: nature’s multifunctional nanoparticles.
ACS Nano, 2016, 10 (3), 3015–3041.
Book chapter
1. Luo L,
Kuai R*, Nanoparticles-assisted peptide cancer vaccine delivery. Cancer Vaccines, Methods in Molecular Biology, published by Springer Nature, 2025, in press.
2.
Kuai R#, Ochyl LJ#, Schwendeman A*, Moon JJ*. Lipid-based nanoparticles for vaccine applications. Biomedical Engineering: Frontier Research and Converging Technologies. 2015, 9, 177-197.



